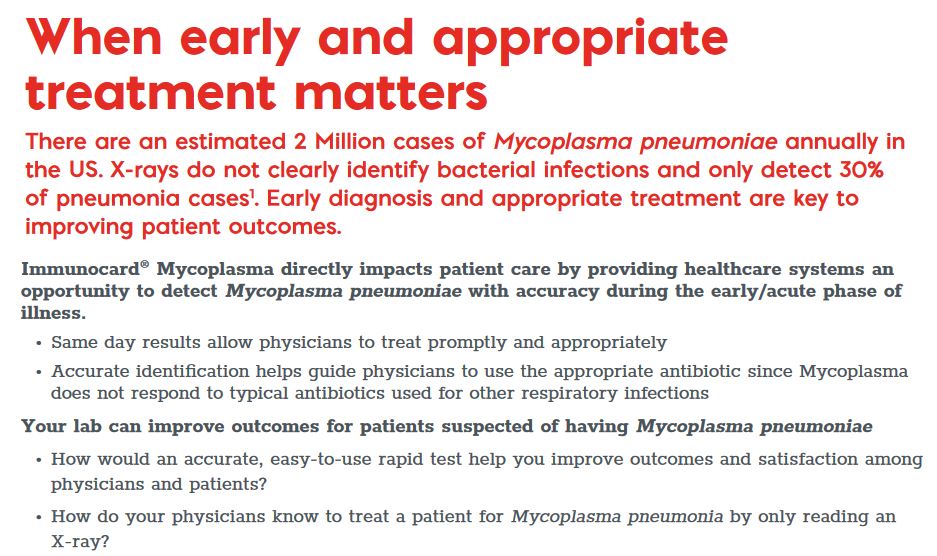
Meridian Bioscience Immunocard Mycoplasma (30 tests/kit) by Meridian Bioscience
Short Description
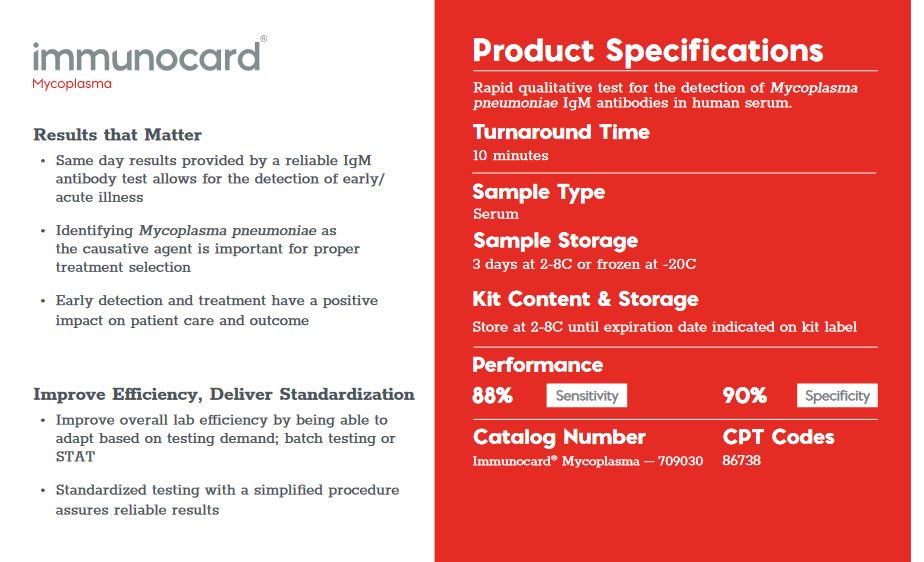
A rapid, sensitive enzyme immunoassay for the detection of IgM to Mycoplasma pneumoniae in human serum.
Share This Item
Meridian Bioscience Immunocard Mycoplasma
The ImmunoCard Mycoplasma enzyme immunoassay (EIA) is an in vitro qualitative procedure for the detection of IgM to Mycoplasma pneumoniae in human serum. Test results are intended to aid in the diagnosis of recent Mycoplasma pneumoniae infection.
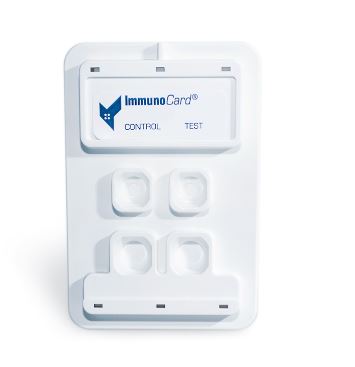
The ImmunoCard Mycoplasma EIA detects the presence of IgM to M. pneumoniae in serum. Patient serum is added to each of the two sample ports. After allowing the sample to enter the device and migrate along the membrane and through the reaction ports, three drops of anti-human IgM alkaline phosphatase conjugate are added to the sample ports and allowed to enter the device.
Three drops of wash and two drops of substrate are then added to each of the reaction ports. Reaction ports are observed for the development of any blue color after five minutes.
The CONTROL port serves as a procedural control, containing immobilized human IgM in the reaction port. The TEST port contains M. pneumoniae antigens and serves as the patient test port.
The development of blue color in the TEST port indicates a reactive test result for IgM to M. pneumoniae. No blue color in the TEST port indicates a nonreactive result.
SPECIMEN:
Preferred sample types:
- Human Serum
Undesirable specimens:
- Specimens with obvious microbial contamination or severe hemolysis should not be tested as they may yield unreliable results.
Collection and Storage:
Serum specimens obtained from clotted blood should be stored at 2-8 C until tested. The specimen should be tested as soon as possible but may be held up to 72 hours at 2-8 C prior to testing. If testing cannot be performed within this time frame, the specimen should be frozen in a non-defrosting freezer (-20 C or lower) immediately upon receipt. Repeated freezing and thawing of specimens should be avoided.
Specimen Preparation:
There are no specimen preparation steps.
MATERIALS AND EQUIPMENT:
MATERIALS PROVIDED:
The maximum number of tests obtained from this test kit is listed on the outer box.
- Test Cards - Individually foil pouched cards containing immobilized detergent extracted M. pneumoniae antigens (TEST reaction port) and human IgM (CONTROL reaction port)
- Positive Control - Sample containing human anti-M. pneumoniae IgM in a buffer containing 0.1% sodium azide
- Negative Control - Buffer containing 0.1% sodium azide
- Enzyme Conjugate - Monoclonal anti-human IgM labeled with alkaline phosphatase in a buffer containing 0.1% sodium azide
- Wash Buffer - Buffer containing 9.5% (weight/vol.) guanidine hydrochloride
- Substrate Reagent - Buffered solution containing 5-bromo-4-chioro-3-indolyl phosphate and 0.1% sodium azide
Standard warranty covered by the seller against any manufacturing defect. In such events, please report to us within 7 days from the date of delivery at connect@lumiere32.sg.
Overall Customer Rating:
0 customer reviews Sign In Or Register to post your reviews. Sign Up0 customer reviews found.
Customers who viewed this item also viewed
Request Quote
Please fill all the details
Write Review
Please fill all the details
Request For Pre-order
Please fill all the details
Request For Product Catalog
Please fill all the details
Request For Sample
Please fill all the details
Request For Sample/Demo
Please fill all the details
Ask More Information
Please fill all the details
Request for Quote/Pre-book
Please fill all the details