SARS-CoV-2 Antigen Self Test Nasal Test Kit, 5 tests kit/box by Quidel
Short Description
Home test kit for Symptomatic and Asymptomatic use
Share This Item
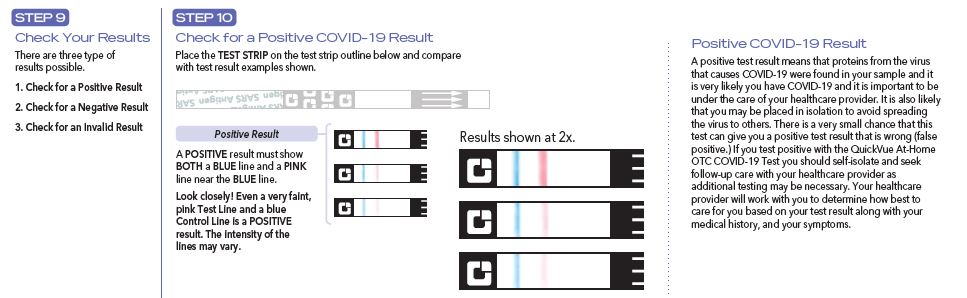
QuickVue At-Home OTC COVID-19 Test
Fast. Easy. Ready When You Are.
QuickVue At-Home OTC COVID-19 Test lets you get rapid results, in the privacy of your own home. Available over-the-counter, everything you need is in the package and taking the test is simple.
The test is authorized for home use with self-collected anterior nasal (nares) swab samples in individuals aged 2 and older. This test is also authorized for home use for individuals aged 2 through 14 with an adult performing the test.
The test is intended to be used twice over two to three days, with at least 24 hours and no more than 36 hours between tests.?
Our test kits also have a shelf life of 24 months from the date of manufacturing, ensuring that you will have access to testing at all times.
How Does the QuickVue At-Home OTC COVID-19 Test Work?
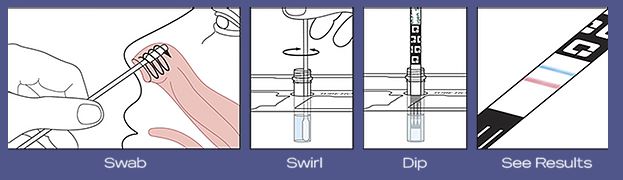
The test uses a gentle self-collected anterior nasal (nares) swab sample to determine a positive or negative COVID-19 result. The swab is swirled in a tube of reagent solution, then removed, before a test strip is inserted. After ten minutes, you can take the strip out of the tube and see your results.
Packaging : 2 tests kit per box
Here is a quick overview of how it works:
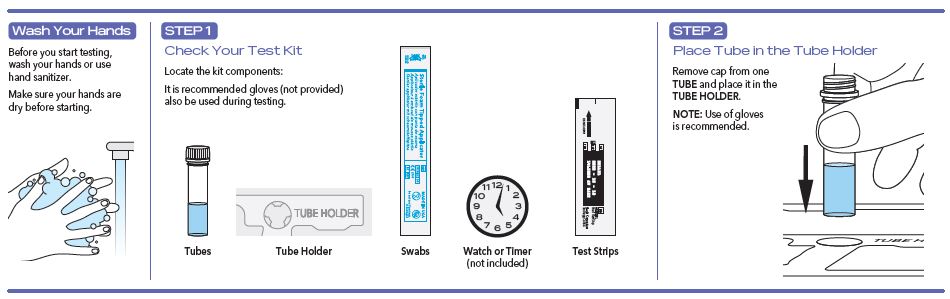
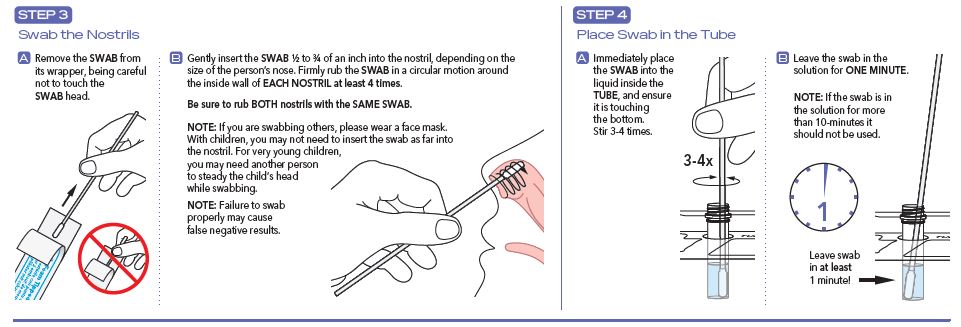
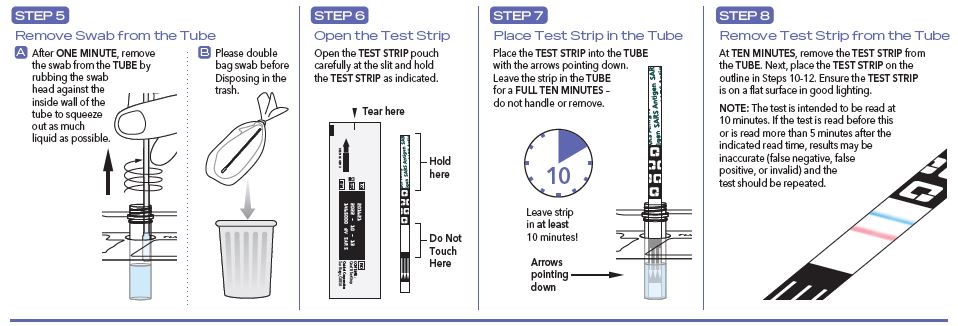
User Instructions
Warnings, Precautions and Safety Information
- The QuickVue At-Home OTC COVID-19 Test is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms of COVID-19. The test is intended to be used twice over two to three days, with at least 24 hours and no more than 36 hours between tests.
- Read the written instructions fully before starting the test procedure
- To ensure correct results, you must follow the instructions
- Keep test kit and materials out of the reach of children and pets before and after use
- Wear safety mask or other face covering when collecting swabs from children or others
- Use of personal protection materials such as gloves are recommended
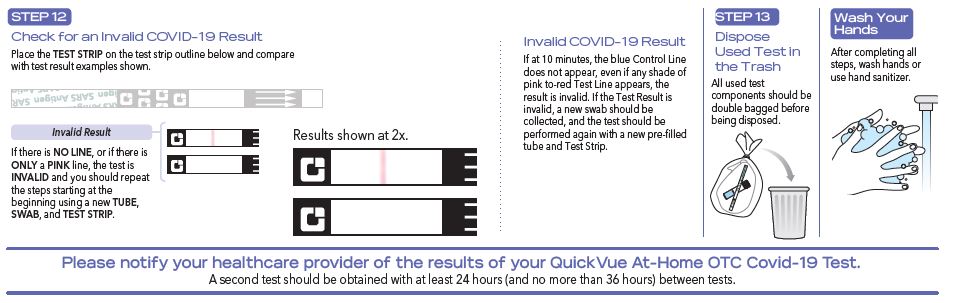
- Do not open the materials until ready for use. If the test strip is open for an hour or longer, invalid test results may occur.
- Improper swab collection may result in incorrectly negative (false negative) results
- The test is intended to be read at 10 minutes. If the test is read before this or is read more than 5 minutes after the indicated read time, results may be inaccurate and the test should be repeated.
- Do not use a test kit that is expired
- Do not touch the swab head when handling the swab
- Avoid exposure of your skin, eyes, nose, or mouth to the solution in the tube.
Intended Use
The QuickVue At-Home OTC COVID-19 Test is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests. This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older.
The QuickVue At-Home OTC COVID-19 Test does not differentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in anterior nasal specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses and the agent detected may not be the definite cause of disease. Individuals who test positive with the QuickVue At-Home OTC COVID-19 Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary.
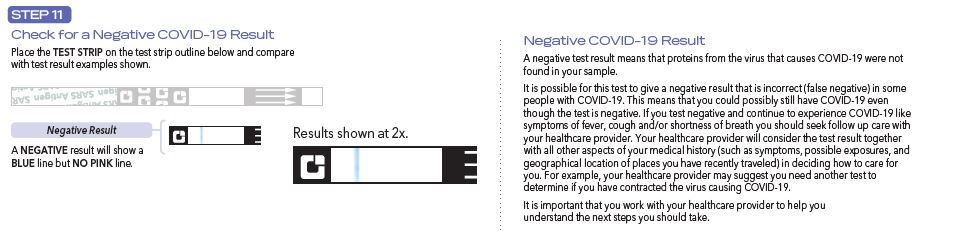
Negative results should be treated as presumptive, do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of an individual’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.
For serial testing programs, additional confirmatory testing with a molecular test for negative results may be necessary, if there is a high likelihood of SARS-CoV-2 infection, such as in an individual with as a close contact with COVID-19 or with suspected exposure to COVID-19 or in communities with high prevalence of infection. Additional confirmatory testing with a molecular test for positive results may also be necessary, if there is a low likelihood of SARS-CoV-2 infection, such as in individuals without known exposures to SARS-CoV-2 or residing in communities with low prevalence of infection.
Individuals who test negative and continue to experience COVID-19 like symptoms of fever, cough and/or shortness of breath may still have SARS-CoV-2 infection and should seek follow up care with their physician or healthcare provider.
Individuals should provide all results obtained with this product to their healthcare provider for public health reporting. All healthcare providers will report all test results they receive from individuals who use the authorized product to relevant public health authorities in accordance with local, state, and federal requirements using appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests provided by CDC.
The QuickVue At-Home OTC COVID-19 Test is authorized for non-prescription self-use and/or, as applicable for an adult lay user testing another person aged 2 years or older in a non-laboratory setting. The QuickVue At-Home OTC COVID-19 Test is only for use under the Food and Drug Administration’s Emergency Use Authorization
Standard warranty covered by the seller against any manufacturing defect. In such events, please report to us within 7 days from the date of delivery at connect@lumiere32.sg.
Overall Customer Rating:
0 customer reviews Sign In Or Register to post your reviews. Sign Up0 customer reviews found.
Customers who viewed this item also viewed
Request Quote
Please fill all the details
Write Review
Please fill all the details
Request For Pre-order
Please fill all the details
Request For Product Catalog
Please fill all the details
Request For Sample
Please fill all the details
Request For Sample/Demo
Please fill all the details
Ask More Information
Please fill all the details
Request for Quote/Pre-book
Please fill all the details